Fuck deBeers
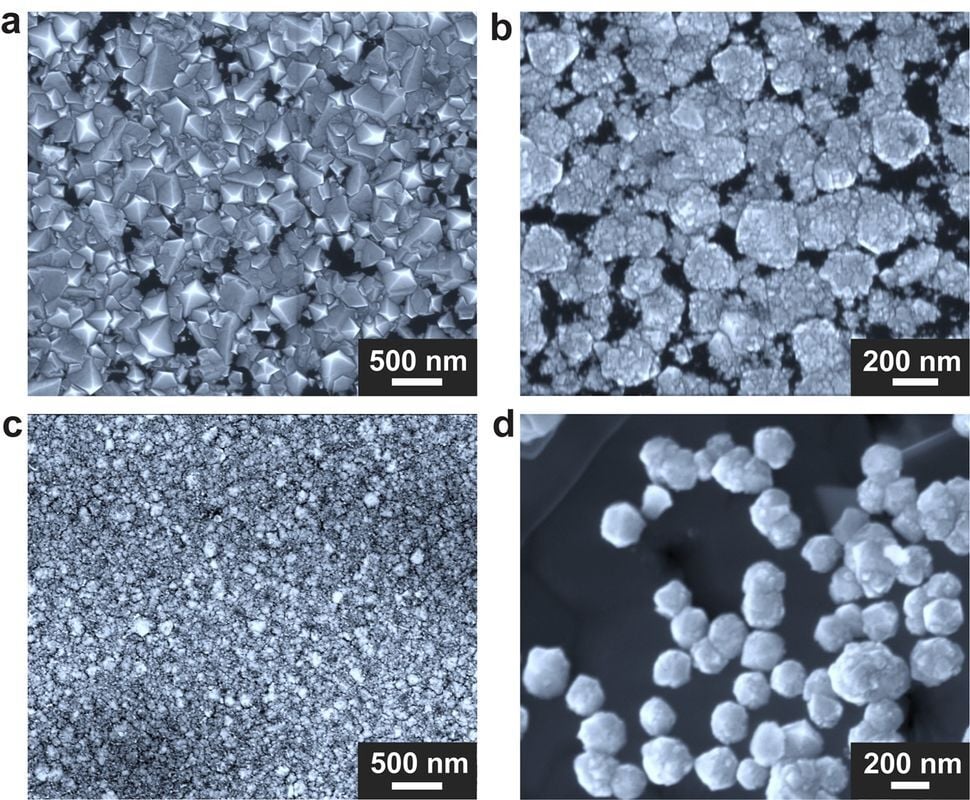
The article shows the diamonds are around 200nm in size.
While not enough for jewelry, this is great for industrial applications, like abrasive grinding wheels or diamond tipped saw blades
I cant wait for all circular saw blades to be diamond.
Trying to size that: That’s 0.2 micrometers. Fine sand is 75 micrometers to 425 micrometers.
(1000 micrometers = 1 millimeter)
This just tells me that diamonds are even more worthless.
Folks like de beers hoard diamonds and jack up prices to make folks think they are more rare that what they really are. We gotta stop the cycle and buy lab grown or use an entirely different stone all together. Diamonds are for basic bitches anyhow
Vintage sapphire is where it’s at. Nothing comes close to the magic blue of untreated sapphire.
Wow, you werent kidding. What a pretty stone.
I got my wife a antique sapphire ring to replace the engagement ring that was stolen in a burglary. When the sun hits that jem, it’s like staring into the deepest clear ocean. I was in the Navy and remember when we were coming to port in Honolulu. The water was the bluest blue I’d ever seen. Old sapphire gives me the same feels
Recommend looking into moissanite also if you like diamonds but don’t want to support the industry. Very similar looking, better in some ways. And because it hardly occurs naturally at all, you can only buy synthetic.
I think quartz looks nice.
Use metal and artistic value, like this.
And if the pattern is open enough, sun ligh will leave the mark on the skin. It’s one very discreet way to keep the “mark” of who we love, skin deep.
So you mean it might be possible to remove the wedding ring without leaving a mark, making it easier to hide that you’re married?
What I wanted to convey is, if the mesh is fine enough, the pattern can get marked on the skin, leaving an elegant but discreet - shall we call it - love brand behind.
If you’re going to cheat, at least be bold enough about it and keep the wedding band on.
Hehe, yeah I intentionally misunderstood your comment to make a joke…
With my so, we actually talked about getting a ring tattoo instead of an actual ring because of how we both never wear jewellery.
Some good humored banter never hurt anybody.
That is the sole thing I draw the line. Scynical as it may sound, ink on skin, no. It feels as an ownership brand that can never be taken off or thrown away.
I personally dislike the notion of being permanent on another life. Either because things don’t work, people grow apart or someone simply dies, from misfortune, sickness or old age, nobody should be tied to another, in any way. Life should go on. Must.
And I’m happily and for a long time monogamous.
They always were – outside of engineering of course.
??? The strongest material available to us seems worthless to manufacture to you??
they clearly meant diamonds as jewelry.
How?
Compared to their artificially inflated price. They’re obviously useful in industry - mainly for their thermal conductivity and their hardness - but their price as a jewel is complete bullshit. They’re not rare at all in nature, but one company controls all of them and uses advertising to drive up demand and public perception.
Wow, I didn’t know that. Interesting.
These diamonds are too tiny for jewelry but I don’t care.
I want a diamond heat spreader for my CPU!
https://www.innovationcooling.com/products/ic-diamond/
You already can.
Go bling up your heat spreading
Everywhere I’m looking says sold out unfortunately. I’ve been using Arctic Silver for over a decade with no issue though, but I’m curious.
Removed by mod
One problem is that the diamonds grown with this technique are tiny
So the next we need is a way to shrink the women so that they fit.
Semaglutide
I’m on semaglutide, it makes me small but unfortunately for the lab-grown diamond industry, I’m still not microscopic.
Everyone always thinks the jewelry when they think of diamonds but I am excited for the prospects of what cheap lab-grown diamonds can do for manufacturing. Diamonds are electrically insulative and yet 10 times more thermally conductive than copper. There are a LOT of industries that would be VERY interested in that.
Hell, it would probably be useful in CPU substrate as well. Instead of silicon semi conductor doping if these could be made precisely enough you could use diamond for the insulation layers and gain that insane heat transfer efficiency to help with avoiding Hotspots. Maybe that’s too thin to matter that much not sure
Heavily used in the building industry too. Concrete saws, tile cutters etc, all expensive as fuck
Diamonds are the hardest mineral known to humans, it’s what we use for all deep sea drilling and excavation.
Removed by mod
We’ll be using that shit to mine asteroids in the next few hundred years or less. Crazy when you think about how far science has been going.
Silicon carbide is much more interesting for the semiconductor industry. With pure carbon there is a lot of lattice mismatch between diamond and single crystal silicon which introduces strain and defects, both of which reduce yield in chip manufacturing.
So you’re saying I could get a diamond tip nozzle for my desktop 3d printer?
You can already, and for quite some time. They claim to be superior to all others.
I have a tungsten steel nozzle on mine and it’s been good for a long time, I imagine it’ll run forever. Does anybody have experience with the diamond ones? Are they worth the extra expense?
I haven’t tried them myself but they have (at least in theory) a lot of benefits speaking for them.
You won’t wear them out, no matter how abrasive your filament is. At least until you print diamonds I guess.
Diamond conducts heat so much better than any other material you might make nozzles from it’s hard to believe. You might (or will) run your prints way cooler, or faster.
Several other things I won’t explain here because I have no idea and would have to make them up on the spot. But how cool would it be to print with a poly crystalline diamond nozzle? I bet you’d drown in panties.
Which could be a drawback.
Looking into it, these are very fragile nozzles. Even more so than the ruby ones. The Tungsten nozzles are the true robust nozzle. It will wear out if you’re using filament with abrasive materials but it takes a lot to do it. I’m going to keep it in mind but probably won’t consider it
The picture is a bit misleading, they are super tiny! Very cool thought.
Looking forward to a future when fake rhinestones are just kinda shit manufactured diamonds.
Super tiny is fine for the things diamonds are used for besides sparkling on fingers.
Similar conditions are employed in the method currently used to synthesize 99% of all artificially created diamonds. Called high-pressure and high-temperature (HPHT) growth, this method uses these extreme settings to coax carbon dissolved in liquid metals, like iron, to convert it to diamond around a small seed, or starter diamond.
Cool. I don’t know how expensive this process is right now, but it seems cheaper to do, at least on mass production.
Edit: I wonder if they could make a tether out of this thing.
“Bender, be careful! Thats the ship’s diamond filament tether. It’s unbreakable!”
“Then why do I have to be careful?”
“It belonged to my grandmother.”
A tether for what?
For everything of course, from space exploration to clothing
Material science.
For a tether, I feel like you’d want something with a high tensile strength, like Kevlar or Zylon. Diamonds are very hard, but also brittle.
Looks like the diamond industry just wrote a new hit list
However, the new method has its own challenges. One problem is that the diamonds grown with this technique are tiny; the largest ones are hundreds of thousands of times smaller than the ones grown with HPHT. That makes them too small to be used as jewels.
Not going to be wearing these any time soon
Just wear hundreds of thousands of them glued together, problem solved.
On a more realistic note though, the applications of this will probably be industrial for a good while. I found it interesting how the article mentions that they were able to develop a diamond coating over their growth substrate. That probably has some cool applications in industrial settings where diamond-plated materials are used.
Diamond saw blades are about to get cheaper
Not in a profit driven economy…
It depends; if a company can use this to make them stupid cheap, then selling them stupid cheap to undercut all their competitors could still make them more money than keeping the price the same and pocketing the saved production costs.
I was making a jab. I’m aware of market forces, but price memory is a thing and often the true cost of production isn’t reflected in consumer pricing. Especially when an industry just decides they can keep prices where they are if not raise them, looking at you egg producers.
Not useful for jewelry, but possibly quite useful for many manufacturing or industrial purposes?
So… useful then?
We have a while to wait before everyone has microdiamonds in their testicles, but one day we’ll get there!
deleted by creator
Do we really want to use the word “groundbreaking” to describe advances in synthetic minerals?
Dad?
Hey hey hey, don’t break the ground. Are they insane?
they are small, but the large diamonds are made from seeds, so still can be used for that, or techniques can improve for larger size production in the future, also, small diamonds are useful for cutting machines
DeBeers says wtf
Either JPL or LM (I can’t remember which) was working on a HTHP system with the goal of being able to grow diamonds with ICs built in. I wonder if this has that potential.
Whut?
Defense contractors want to grow diamond computer processors. Because silicon breaks down at high temperatures.
Don’t diamonds burn? They are just carbon.
Ah, I just assume as it was carbon it would still be quite low (relatively)
Carbon, compressed hard enough it becomes diamond, forms a square crystal lattice that is so crazy stable that it’s very resistant to change (including from burning).
Is diamond more resistant to burning than other forms of carbon? I thought even lowly graphite is pretty stable at high temperatures…
Just like humans are basically just trees, because they’re both carbon based lifeforms, right?
Na, we are bags of water for the purposes of most approximations
Yeah, but at about 400 degrees higher than silicon.













