Good for them, I guess, but those of us with frequent respiratory infections knew that phenylephrine is useless for at least a decade by now.
Is this in addition to rebound congestion or aside from it?
Not to doubt Scientific American, but phenylephrine works. It’s the only thing that clears my sinuses when they get blocked. They’re talking about it not being a nasal decongestent, but I don’t think it’s suppossed to be?
There’s no good evidence phenylephrine works for clearing up your nasal passages, at least at the doses sold over the counter. Nasal decongestant is what it’s labeled to be sold as, you’re referring to the same idea just with different words. Study after study has failed to show any difference between phenylephrine and placebo. In most studies both groups report getting somewhat better, there’s just no difference to placebo. Suggesting improvements are just due to some combination of time and placebo effect.
https://pubmed.ncbi.nlm.nih.gov/26143019/
That’s just one but there’s many others.
Pseudophedrine on the other hand has been shown to be superior to placebo many times (again just one source below, but there are others):
https://pubmed.ncbi.nlm.nih.gov/15794071/
That one is still available. It’s just kept behind the counter because of concerns it could be used in the manufacture of meth.
I’ve not personally seen it sold as a nasal decongestant. It’s in my sinus meds. But saying it doesn’t work is false, it doesn’t work for a very specific use. It actually has quite a few uses beyond congestion.
The study you quote is into it’s use in “Nasal Congestion in Seasonal Allergic Rhinitis” which is even more specific than the article is.
It’s labeled as a nasal decongestant (your description of “opening up the sinuses” would fall into this, we’re talking about the same thing here, just different words), that is the indication it got for sale over the counter by the fda. It’s literally not allowed to be sold over the counter in its oral form for any other purpose by fda regulations (unless you count the topical form for hemorrhoids). Marketing a drug for unapproved uses is illegal.
I posted that study as an example, feel free to research on your own to look at the many negative studies in many similar situations showing it doesn’t work for this purpose. Here’s a meta analysis to save you some time:
https://pubmed.ncbi.nlm.nih.gov/17264159/
The lack of evidence is why the fda pulled it, and why you’ll no longer be able to buy it over the counter in its oral form for the purposes of nasal decongestion.
It has other purposes of course, like being used intravenously to help increase blood pressure in the icu, or in the ophthalmology office to dilate pupil. But that’s not something you need over the counter. Taking it in it’s oral form at the over the counter dose to aid with nasal decongestion has no good evidence. I wasn’t implying it’s never used for anything else. That’s its only over the counter indication for its oral form I’m aware of. I think there’s a topical form in some hemorrhoid creams and the nasal spray form. The fda pull doesn’t affect any of its other uses.
Pseudophedrine is an approved over the counter nasal decongestant, and is still available (and has evidence backing it up for this use unlike phenylephrine). There’s also a nasal spray decongestant (afrin), but that can have some serious drawbacks if overused. Definitely read the packages on all of them carefully. I think the nasal spray topical form of phenylephrine might still be available though. All of the studies above refer to the commonly sold oral version.
I can’t tell if the other poster is being intentionally obtuse or is genuinely ignorant of the science.
Nasal means nose. Your sinuses are not your nose. Not sure why this is difficult? You’ve posted a second link to its use as a nasal decongestant.
“MEDLINE, the Cochrane Central Registry of Controlled Trials, EMBASE, International Pharmaceutical Abstracts, and the Federal Register were searched for English and non-English-language studies published through January 2007 that measured the effects of oral phenylephrine on nasal airway resistance (NAR) in patients with nasal congestion.”
Again, you’re making a distinction where there is none, we are talking about the same thing. So you’ll never find a study with the specific term you want, because that’s not the term used for that symptom in research.Your sinuses are a part of your nasal passage and nasal airway (nasal doesn’t just mean, literally in the nose in the front of your face and nowhere else in this context, it’s referring to the whole cavity), decongestants consrict blood vessels to shrink the size of tissues and to reduce production of mucus. There’s no such thing as an over the counter medication with a specific indication of “sinus congestion” versus “nasal congestion.” Phenylephrine never carried any special label as a “sinus” decongestant, that’s not an actual distinction anyone makes, it’s the same tissue. It’s like you’re saying “sure you have all these studies about global warming, but I was asking about climate change,” like yes, these refer to the same phenomenon.
Like here’s a label if you don’t believe me:
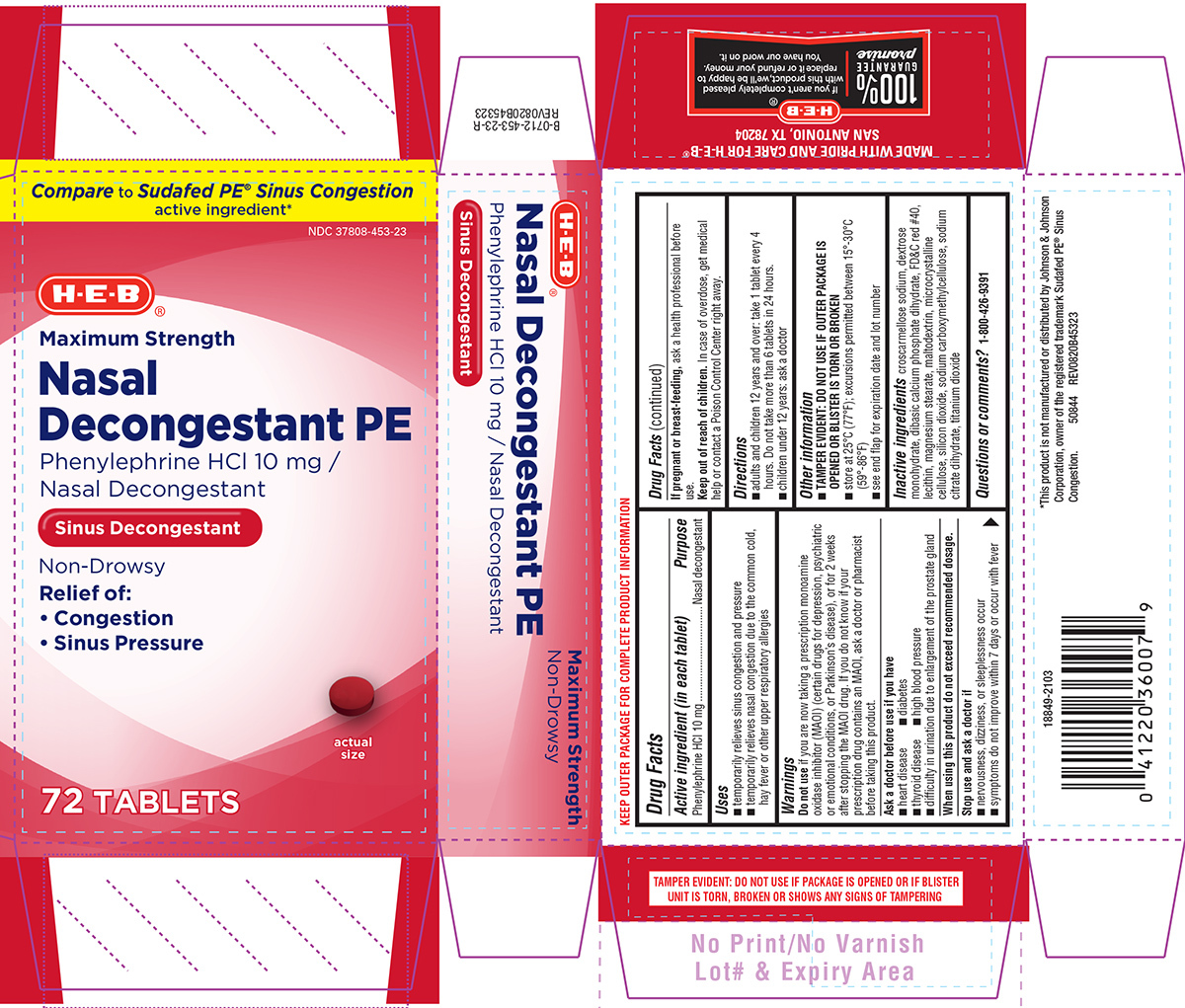
Look at the box of active ingredients: it says nasal decongestant (not sinus decongestant) is the indication. It refers to your whole nasal airway, including sinuses. That’s why under uses afterward it specifically mentions sinus pressure/symptoms. Nasal decongestant refers to relieving that symptom. This is my last comment on this chain because I don’t know what else to tell you. You’re misinterpreting terms here. And if you still don’t believe me, if the nasal decongestant indication didn’t refer to relieving sinus pressure, it would literally be illegal for it to be labeled this way, as drugs cannot be labeled for a non-FDA approved indication. But don’t worry there’s still plenty of alternative decongestants that do have actual evidence that can be used.
This is a whole “Shit Americans Say” conversation. I understand that you are struggling with the idea that meds can be used for other reasons, reasons that may not be allowed in your country.
However the headline saying a medication doesn’t work is false.
You have linked studies about breathing, do you breathe through your sinuses?
https://www.saintlukeskc.org/health-library/understanding-nasal-anatomy-inside-view
Here is a diagram to help, it labels the nasal cavity and the sinuses which it shows you are air filled chambers around your nose.
Are you brain damaged or something?
The oral absorption of phenylephrine is erratic. Perhaps that is why it was not used as an oral decongestant until it was the only choice. It had long been known that enzymes in the gut lining metabolized oral phenylephrine to inactive metabolites, reducing the amount of the active compound that could enter the bloodstream.
The most cited study found that an oral dose of phenylephrine had an absorption rate of 38 percent of an oral dose of phenylephrine, but this study measured more than just the compound’s active form. Later studies with more sensitive tests found that less than 1 percent of oral phenylephrine enters the bloodstream in an active form.
You stand alone against many pharmacist who study the medicine, research scientists who test the medicine, and pharmaceutical labs that make the medicine. The only people who want to continue making phenylephrine are those who make hundreds of millions in the misconception it will help you. That’s stealing, even if no one gets hurt.
You are probably very sensitive to phenylephrine so it works with those very small amounts. Everyone else is not you. You are lucky to find a solution on the shelf right now. Overwhelmingly we others cannot. It is this very limited helpfulness that has caused phenylephrine to lose its GRASE status (Generally Recognized as Safe & Effective) since it is safe but not broadly effective.
I think you’re confused. The people quoted here say it does not work in one very specific use which as far as I can see it isn’t marketed for in most countries. Doesn’t appear to be in mine.
This publication has written a pop science headline claiming it doesn’t work at all.